Research News
Strategies and challenges for the next generation of cancer targeting?drug delivery system
 Updated: Jan 30, 2019
Updated: Jan 30, 2019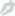 Written:
Written:  Edited:
Edited:
Written by: Hospital of Stomatology
Edited by: Wang Dongmei
Recently, Professor Hua Wang from the Hospital of Stomatology, Professor Yan Zhang from the School of Life Sciences, Sun Yat-sen University and their students published their research titled “New Cell-Penetrating Peptide (KRP) with Multiple Physicochemical Properties Endows Doxorubicin with Tumor Targeting and Improves Its Therapeutic Index” in the ACS Appl. Mater. Interfaces (2019, XX-XX, IF 8.097).
Conventional cytotoxic anticancer drugs usually only have a small fraction of the drugs that reach the tumor tissue to play an anticancer role after entering the blood circulation. This is the fundamental reason that restricts the efficacy of drugs and leads to their toxic and side effects. It is a dream of human beings and the ultimate goal of drug development to develop anti-cancer drugs with the ability of precise targeting like missiles.
Recently, they developed a tumor-targeted drug delivery system (DDS) by linking KRP and doxorubicin (DOX) with stable covalent bonds (thioether bond and amide bond). They demonstrated that the multiple physicochemical properties of KRP endow KRP-DOX with multiple synergistic functions, including good biocompatibility and biodistribution, selective accumulation in tumor tissues, inclination to remain in tumor tissues and be internalized by tumor cells; stable covalent bonds prevent free DOX release from KRP-DOX in blood stream, shield normal tissues from the toxic effect of DOX, and lead to the majority of DOX delivery into tumor cells by KRP; lysosome escape of KRP-DOX ensures its tumor-killing effect.
Since 2000, the approvals of gemtuzumab ozogamicin have paved the way for ongoing clinical trials that are evaluating more than 60 further ADC candidates. However, cell-penetrating peptides drug delivery system such as TAT still stay at the door of clinical application because lack of targetability and more susceptible to enzymatic hydrolysis. KRP-DOX can modify the biodistribution model of the small molecule DOX, i.e., replace the simple diffusion into many organs with selective accumulation in solid tumors by the EPR effect since KRP-DOX has many positive charges, which can bind to tumor cells by electrostatic interaction with the negative charges on tumor cell membranes. KRP-DOX can be efficiently internalized by tumor cells and can escape from endolysosome.
Compared to therapeutic antibodies, KRPs are artificial synthesized, have simple structure and better tissue penetration. They are relatively safe for human application because of their complete bicompatibility and fewer adverse effect associated with complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity.
This work was supported by a grant from the National Natural Science Foundation of China (grant no. 31371390), National High-tech R&D Program (863 Program) of the Ministry of Science and Technology of China (No. 2014AA020702), and two Programs of Guangdong Science and Technology Department (Nos. 2016B030231001 and 2017B020230002).
Link to the article: https://pubs.acs.org/doi/pdf/10.1021/acsami.8b21027



