Source: Sun Yat-sen Memorial Hospital
Edited by: Zheng Longfei, Wang Dongmei
Immunotherapy is revolutionized achievement for tumor therapy. At present, the mainstream immunotherapy is to promote the cytotoxicity of T cells to cancer cells. Inducing immune cells to phagocytize cancer cells has become an important idea of the next generation immunotherapy. Many monoclonal antibodies can induce macrophages to phagocytize cancer cells (1), and their mechanisms includes: 1. Fc gamma receptor (FcgR)-mediated phagocytosis, which is called as antibody-dependent cellular phagocytosis (ADCP), such as Trastuzumab and Rituximab (2). 2. The “don’t eat me signal, such as CD47 monoclonal antibodies (3). However, the effect of antibody induced tumor phagocytosis is not ideal in the clinical application, especially in solid tumors. Therefore, clarifying how macrophages effectively phagocytize cancer cells is of great significance for the design of next-generation tumor immunotherapy.
Su Shicheng team from Sun Yat-sen Memorial Hospital published a research achievement entitled “Macrophage mitochondrial fission improves cancer cell phagocytosis induced by therapeutic antibodies and is impaired by glutamine competition” in
Nature Cancer on April 28, 2022. The article discovered that mitochondrial fission promotes macrophages to phagocytize cancer cells by changing the phase transition of WIP and WASP, two important components of phagocytosis machine. Enzymes that target glutamine competition in the tumor microenvironment can improve the efficacy of multiple monoclonal antibodies by promoting tumor phagocytosis.
Mitochondria provide energy for immune response (4). The authors discovered circle RNA transcribed by mitochondrial gene regulates fibroblast function by ROS in previous reports (Bioart:Cell-Su Shicheng, Gao Zhiliang and Xu Xiaoding were cooperatied to reveal the mechanism of mitochondrial localized circRNA regulating liver immune metabolic inflammation) (5). The authors want to know that whether the macrophage phagocytosis to tumor cells is regulated by mitochondrial dynamic. When Trastuzumab, Rituximab and CD47 monoclonal antibodies were cocultured with 18 cell types of breast cancer, colon cancer and B cell lymphoma, respectively. The mitochondria of macrophage phagocytized tumor cells remain fission condition as granular and Drp1, a protein regulated mitochondrial fission, is substantially increased. Macrophage fission inhibition by gene deletion or pharmaceutical interrupt phagocytosis ability to tumor cells. Macrophages from wild-type mice were transferred into Csf1op/op tumor burden mice which luck macrophages, promotes antibody-induced phagocytosis to tumor cells and therapy responses, but not the macrophages with Drp1 knockdown. Confirming that mitochondrial fission is necessary for macrophages to effectively phagocytize tumor cells In vitro and in vivo experiments (Fig. 1).
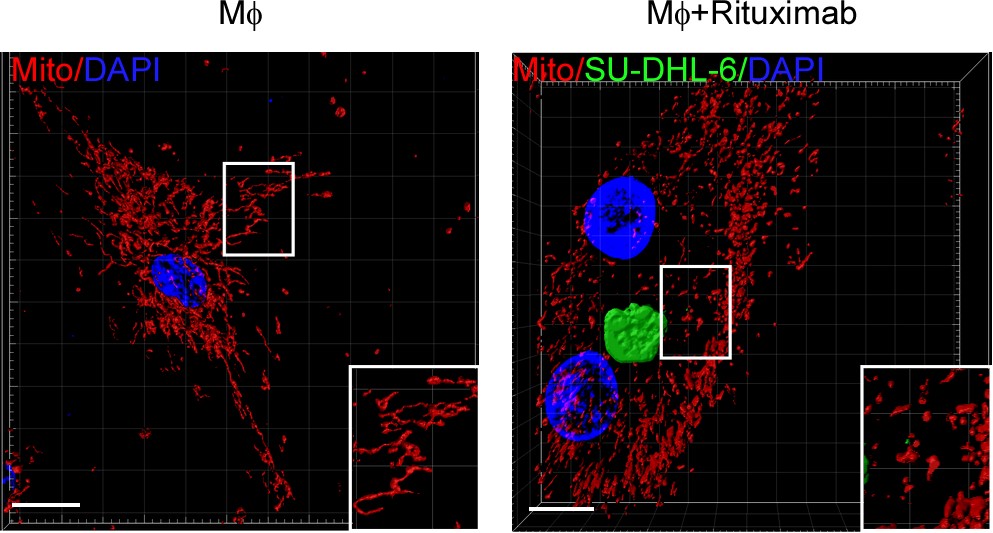
Figure 1: mitochondrial fission of macrophage promotes phagocytosis to tumor cells
Actin polarization is a main step to machinery regeneration in large substances phagocytosis (6). The authors discovered that mitochondrial fission inhibition interrupt actin polarization. Multiple studies have shown that the aggregation of intracellular proteins (liquid-liquid phase separation) can regulate the functions of a variety of cells (7,8). The authors analyzed protein structure by IUPred2A website and found WIP and WASP, important contents for phagocytosis machinery, contain large intrinsically disordered regions (IDRs) that facilitate condensate formation. The authors demonstrated that WIP and WASP can form droplets by droplet formation in vitro, bleaching and fluorescence colocalization experiments. To explore the mechanism of mitochondrial fission and phase separation of WIP and WASP. The authors checked the products change of mitochondrial fission, and the influence in phase separation of WIP and WASP to the change. The increased levels of Ca
2+ from mitochondrial fission inhibits phase separation of WIP and WASP in experiment results. In further experiments, the authors discovered that phase separation of WIP and WASP inhibits WIP phosphorylation by PKC-θ. High Ca
2+ levels induced by mitochondrial fission promotes WIP attachment and phosphorylation with PKC-θ by interrupting WIP/WASP droplets, and then enhances the actin polarization of macrophage and phagocytosis to tumor cells.
To explore the relation of tumor cells resistant to antibody-induced phagocytosis and mitochondrial fission of macrophages. The authors checked the differential protein levels between resistant- or sensitive-tumor cells by microarray, Western blot, mass spectrometry and Ocomine database. They found that GFPT2, a limited enzyme for hexokinase pathway, is highly expressed in tumor cells resistant to phagocytosis, which accelerate metabolism products of GFPT2. GFPT2 is a rate limiting enzyme regulating the decomposition of glutamine into glutamate, which can promote the consumption of glutamine, and the consumption of glutamine can inhibit the fission of mitochondria (9). Tumor cell GFPT2 knockdown or adding GFPT2 substrate-glutamine increase the fission of macrophage mitochondria and phagocytosis of tolerant tumor cells, while removing glutamine in the microenvironment inhibited the phagocytosis of sensitive tumor cells. Gene knockdown and chemical inhibition of GFPT2 enzyme can improve the effect of clinical monoclonal antibody in tumor treatment.
Conclusion, through the study of tumor phagocytosis models mediated by different antibodies, revealing that the competition of glutamine in the tumor microenvironment inhibits mitochondrial fission of macrophages, maintains WIP / WASP phase separation and inhibits that PKC-θ phosphorylated WIP and actin polarization, eventually produces clinical antibody resistance. This study provides a new idea for enhancing the effect of next-generation tumor immunotherapy.
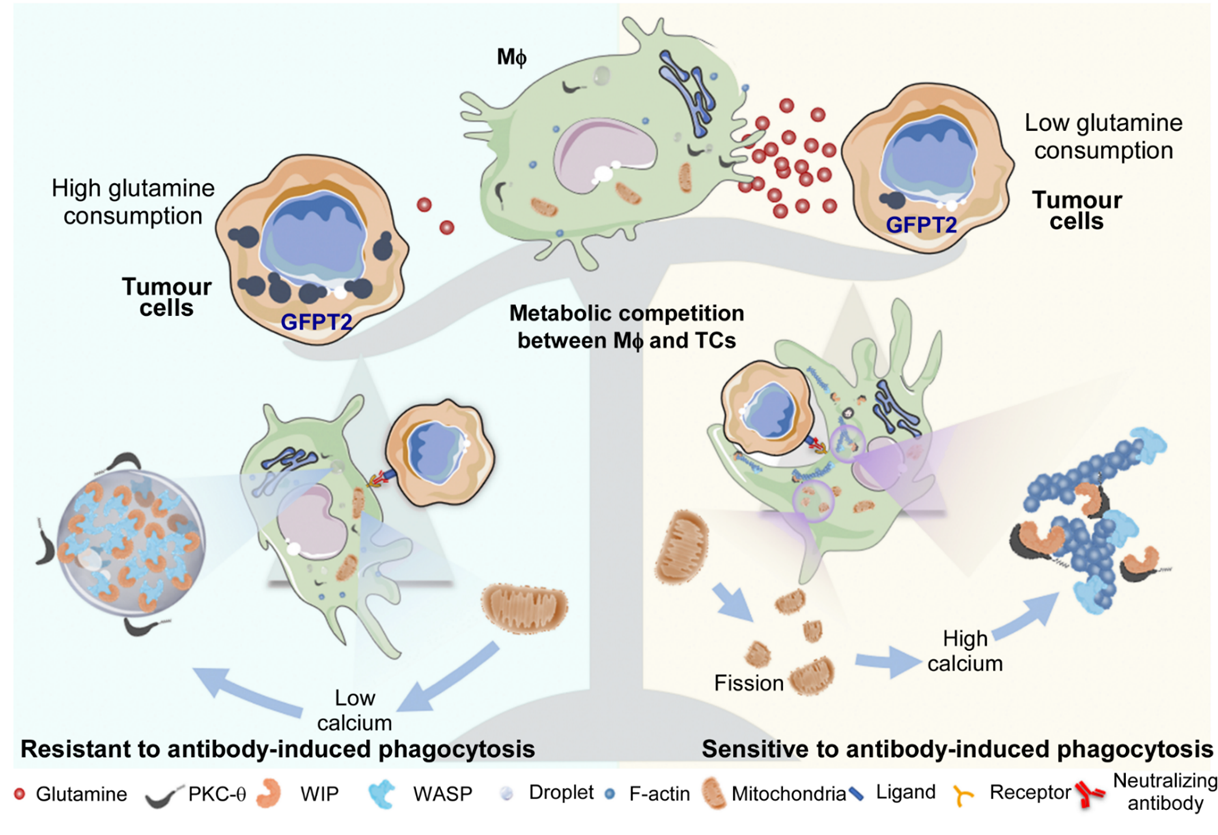
Figure 2: the main mechanism graph in this article
Professor Su Shicheng is the corresponding author of this article, and postdoctoral Li Jiang, doctoral students Ye Yingying, Liu Zhihan and Dr. Zhang Guoyang are the first co-authors of this article. Professor Cai Qingqing, Professor Ma Liping and Professor Feng Ru provided strong support and help for this article.
Link to the article:
https://www.nature.com/articles/s43018-022-00354-5



