Recently, Professor Zhengqi Lu's group from the Department of Neurology, Mental and Neurological Disease Research Center, the Third Affiliated Hospital of Sun Yat-sen University, published the latest original research entitled “FOXP3+ macrophage represses acute ischemic stroke-induced neural inflammation”in Autophagy. The study found that FOXP3+ macrophages suppressed acute ischemic stroke-induced neural inflammation.
Acute ischemic stroke (AIS) is a serious disease with a high disability and mortality rate and is widespread worldwide. Abrupt interruption of brain blood flow in AIS results in neuronal death and the subsequent neural inflammation, which further exacerbates stroke outcomes. Therefore, inflammatory resolution represents a favorable therapeutic strategy against AIS.
Macrophages play a vital role in the pathophysiology of ischemic stroke. Macrophages respond rapidly to cerebral ischemia and numerously infiltrate into stroke lesions. At the same time, macrophages terminate cell-death-induced neural inflammation and accelerate homeostasis restoration in AIS by clearing injured cells or debris, which is also known as efferocytosis. However, the molecular mechanisms of macrophage efferocytosis remain to be explored. Notably, FOXP3 is the master immune repressive transcription factor of regulatory T cell (Treg). Accumulating evidence suggest that FOXP3 expression is not limited to T cells. Expression and molecular function of FOXP3 in macrophages are elusive.
The study investigated the role of FOXP3 in macrophags during AIS with myeloid specific Foxp3 knockout mice (Lyz2Cre-ERT2Foxp3fl/Y). Cerebral ischemia was induced with transient middle cerebral artery occlusion (tMCAO). FOXP3+ macrophages were detected in the peri-infarct region of stroke brain. Single-cell RNA sequencing (scRNAseq) and FOXP3-ChIP revealed that FOXP3+ macrophages were actively involved in the clearance of dead cells and debris in stroke lesions. FOXP3+ macrophages showed enhanced efferocytosis and anti-inflammatory efficiency.
To investigate the mechanisms by which FOXP3 regulates macrophage’s functions in AIS, FOXP3 binding protein in BMDM was isolated with immunoprecipitation (IP), then subjected to liquid chromatography-mass spectrometry (LC-MS) analysis and the subsequent GO-BP projection. It was found that, in resting BMDM, Foxp3 was stably and sparingly expressed, but continuously degraded through autophagy. LC3-associated phagocytosis (LAP) was initiated in macrophages after stroke, which competed autophagic machineries that were responsible for FOXP3 protein degradation. FOXP3 protein was thus rapidly accumulated and translocated into the nucleus to promote phagocytic activities and subsequent cargo digestion in macrophages, which terminated the dead cells induced immune responses and contributed to inflammatory resolution after ischemic stroke. These evidence suggest that FOXP3 signalling is subtly and exquisitely controlled by a complex molecular network which rapidly fine-tune macrophage activity in AIS.
In conclusion, the study elucidates that FOXP3+ macrophages are implicated in post-stroke neural inflammation. FOXP3 positively regulates phagocytosis and the inflammatory resolving functions of macrophages. Rapid activation of FOXP3 in macrophages is associated with LAP. The research team proposed that FOXP3 could be a viable molecular target for cultivating efferocytosis and inflammatory resolution of macrophages in the ischemic brain, which provides a new theoretical basis and intervention strategy to mitigate brain injury and promote stroke recovery.
Corresponding authors of the study are Prof. Zhengqi Lu (Mental and Neurological Disease Research Center, the Third Affiliated Hospital of Sun Yat-sen University), Prof. Yan Lu (Center of Clinical Immunology, the Third Affiliated Hospital of Sun Yat-sen University) and professor Quentin Liu (Sun Yat-sen University Cancer Center). First authors include Prof. Wei Cai and PhD candidate Mengyan Hu (Mental and Neurological Disease Research Center, the Third Affiliated Hospital of Sun Yat-sen University).
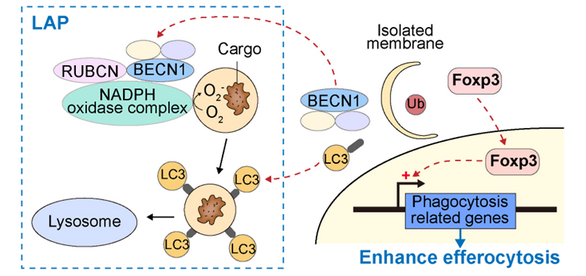
The role of FOXP3 in macrophage during AIS
Link to the paper: https://doi.org/10.1080/15548627.2022.2116833



