Aerobic glycolysis is considered a hallmark feature of cancer. Recently, Prof. Bin Wu's team from the Third Affiliated Hospital of Sun Yat-sen University published an original article entitled "Aerobic glycolysis enhances HBx-initiated hepatocellular carcinoma via NF-κBp65/HK2 signaling" online in the Journal of Experimental & Clinical Cancer Research on Nov. 21th, revealed a key mechanism of HBx enhances aerobic glycolysis via the NF-κBp65/HK2 pathway, further increasing hepatocyte proliferation and resulting in hepatocellular carcinogenesis.
Aerobic glycolysis has been recognized as one of the growth-promoting metabolic alterations of cancer cells. Emerging evidence indicates that nuclear factor κB (NF-κB) plays a significant role in metabolic adaptation in normal cells and cancer cells. However, the mechanism of NF-κB regulates metabolic reprogramming in hepatocellular carcinoma (HCC), specifically hepatitis B virus X protein (HBx)-initiated HCC, has not been determined. Therefore, it is of great significance to elucidate the regulatory mechanism of NF-κBp65 on aerobic glycolytic metabolic reprogramming in HBx-initiated HCC.
Prof. Wu's team has successfully constructed a new HBx+/+/NF-κBp65f/f and HBx+/+/NF-κBp65Δhepa spontaneous HCC mouse model. From in vivo and in vitro assays, the team found that NF-κBp65 was upregulated in hepatitis B virus (HBV)-related HCC (Figure2), and HBx induced NF-κBp65 overexpression at the transcriptional level and promoted its phosphorylation by the post-translational modification. Hepatocyte-specific NF-κBp65 deficiency remarkably decreased HBx-initiated spontaneous HCC incidence in HBx-TG mice. Mechanistically, HBx induced aerobic glycolysis by activating NF-κBp65/hexokinase 2 (HK2) signaling in spontaneous hepatocarcinogenesis, and overproduced lactate significantly activated the PI3K (phosphatidylinositide 3-kinase)/Akt pathway further promoted the pernicious proliferation of hepatocytes and eventually led to carcinogenesis. Inhibition of NF-κBp65 in hepatocytes decreased pernicious proliferation by downregulating aerobic glycolysis.

Figure 1. NF-κBp65 was upregulated in HBV-associated hepatocellular cancer.
This research clarifies a vital mechanism of aerobic glycolytic metabolic reprogramming in the carcinogenesis of HBV-related HCC, and reveals that HBx expressed by HBV can promote the occurrence and development of HCC through NF-κBp65-mediated aerobic glycolysis. These findings provide a novel theoretical basis for the prevention and treatment of HCC, and suggest potential therapeutic targets (Figure 2).
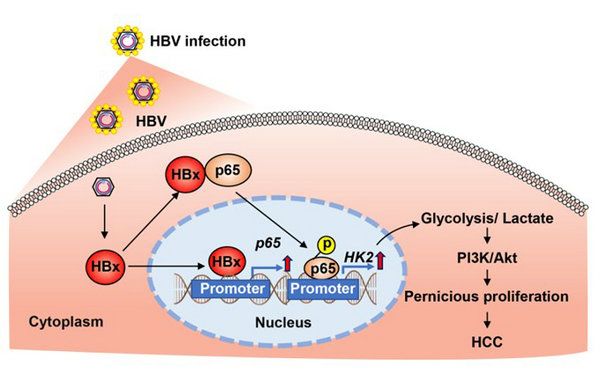
Figure 2. HBx promotes the expression and activation of NF-κBp65 in hepatocytes and enhances aerobic glycolysis via the NF-κBp65/HK2 pathway to overproduce lactate, further increasing hepatocyte proliferation through PI3K/Akt signaling and resulting in hepatocellular carcinogenesis.
This work is a further expansion and in-depth study based on the previous studies published in the Nat Commun, Autophagy, and J Exp Clin Cancer Res. Prof. Wu from the Third Affiliated Hospital of Sun Yat-sen University is the corresponding author, and his PhD candidate Lingjun Chen is the first author. This work was supported by grants from the Natural Science Foundation Team Project of Guangdong Province (2018B030312009) and the National Natural Science Foundation of China (82070574, U1501224).
Link to the article: https://jeccr.biomedcentral.com/articles/10.1186/s13046-022-02531-x



