Research News
Ming Kuang's team first revealed the unique tumor immune microenvironments of two different types of recurrent hepatocellular carcinoma in the journal Gut
 Updated: Jan 19, 2023
Updated: Jan 19, 2023 Written:
Written:  Edited: Tan Xi, Wang Dongmei
Edited: Tan Xi, Wang Dongmei
On January 3rd, 2023, the research finding “Distinct Single-cell Immune Ecosystems Distinguish True and De Novo HBV-related Hepatocellular Carcinoma Recurrences” was published online in Gut (IF=31.79), the Journal of the British Society of Gastroenterology and the top journal in the field of gastroenterology, by Professor Ming Kuang's team from the Center of Hepato-Pancreato-Biliary Surgery, The First Affiliated Hospital of Sun Yat-sen University. This research provided the first in-depth analysis of the tumor immune microenvironment (TIME) in two types of hepatocellular carcinoma (HCC) recurrence patterns and revealed the unique immune response related to the true or de novo HCC recurrences, thus providing immunotherapy guidance and the scientific basis for the precise prevention and treatment of different types of recurrent HCCs.
HCC is the third most common cause of cancer-related death worldwide. Radical surgery is considered an effective treatment for HCC. However, the recurrence rate is high, reaching approximately 70% within 5 years, and seriously affects the long-term prognosis of HCC patients. The diagnosis and treatment of recurrent HCCs is a clinical difficulty. However, the current treatment is often based on the molecular and pathological characteristics of primary HCCs, and there is a lack of high-quality research that provides scientific evidence for the treatment and management of recurrent HCCs. In recent years, immune checkpoint inhibitors have shown a certain therapeutic potential in HCC, but less than 30% of the patients have benefited. One of the important reasons is the complex tumor immune ecosystem of HCC. Therefore, a comprehensive and in-depth analysis of the immune microenvironment atlas of recurrent HCC is crucial to developing personalized immunotherapeutic strategies and improving the prognosis of HCC patients. There are generally two types of HCC recurrence patterns. One is true recurrence (caused by primary tumor dissemination), and the other is de novo cancer (newly arising from the damaged liver parenchyma), which has little relationship with the primary tumor. At present, it has not been reported whether and what kind of differences exist in the tumor immune ecosystems of these two recurrent HCCs.
To analyze the characteristics of tumors and microenvironments in different types of recurrent HCCs. Researchers collected 23 tumor samples and 11 paired non-tumor adjacent liver tissues (NAT) from 20 treatment-na?ve recurrent HCC patients (discovery cohort) and performed scRNA-seq and bulk RNA- seq. Moreover, a validation cohort 1 (26 patients with paired surgical samples of the primary and recurrent HCC) for bulk RNA- seq and a validation cohort 2 (47 patients with paired primary and recurrent surgical samples) for WES were included. In each of the three cohorts, WES data for paired primary and recurrent HCC were used to determine the types of recurrence patterns (true recurrence vs de novo recurrence).
According to the sequencing results, researchers found that, contrary to the traditional clinical knowledge, de novo HCC recurrences could often occur earlier than 2 years after resection. Moreover, early dissemination of primary HCC in the liver parenchyma resulted in true recurrence when primary lesions were <1 cm in diameter. Therefore, radical surgery alone is not enough to prevent tumor recurrence. These results suggest the necessity and importance of neoadjuvant or adjuvant therapy in preventing postoperative HCC recurrences.
Next, researchers found significant differences in TIME between true and de novo HCC recurrences. The TIME of truly recurrent HCCs was characterized by reduced frequency and function of dendritic cells (DC) and increased abundance of KLRB1+CD8+ T cells with memory phenotype and low cytotoxicity. In contrast, higher proportions of DCs and enrichment in cytotoxic and exhausted CD8+T cells were found in the TIME of de novo recurrent HCCs. By Monocle, the dynamic immune states and cell transition trajectories of tumor-specific CD8+T cells in the two recurrent HCC types were inferred. It showed that most effector CD8+T cells in de novo recurrent HCCs could transit to exhausted CD8+T cells, while a significant part of effector CD8+T cells in truly recurrent HCCs transit to KLRB1+CD8+T cells with immunosuppressive function and low cytotoxicity. Further, researchers tried to uncover the reasons for the differences in microenvironment between these two types of recurrent HCCs. HCC cells in truly recurrent HCCs had a higher immune escape ability than those in de novo recurrent HCCs. AND in-depth analysis showed that the former highly expressed the immunosuppressive molecule GDF15, which may have dampened antigen presentation of DC through the GDF15-CD44 axis and inhibited the antitumor activity of CD8+T cells. Besides, the high expression level of GDF15 was closely related to the recurrence of primary or recurrent HCC. In addition, the strong interactions between myeloid cells and CD8+T cells in de novo recurrent HCCs induced exhausted CD8+T cells and mediated immunosuppressive microenvironment through PD1/PD-L1 and Galectin9/Tim3 axis. These results suggest that true and de novo HCC recurrences may require different immunotherapy strategies. Truly recurrent HCCs may require therapy to stimulate the release of tumor antigen in combination with anti-GDF15 therapy, while de novo recurrent HCCs may benefit from immune checkpoint inhibitors. At the same time, researchers also conducted a phase II clinical trial of neoadjuvant anti-PD-1 therapy for resectable recurrent HCCs, and the preliminary results showed more responses in de novo recurrent HCC patients, which supports the results of sequencing and experimental above.
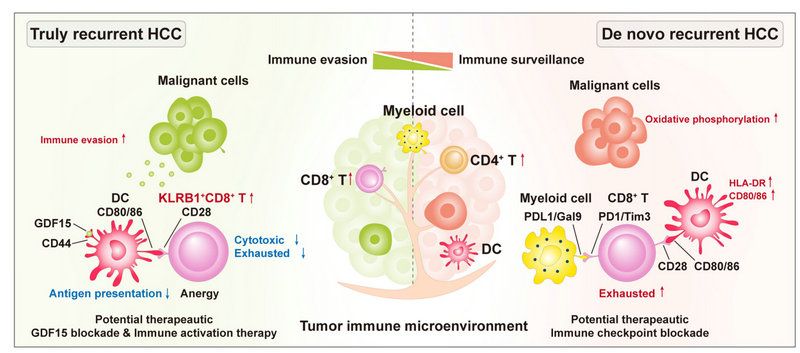
Figure. Diagram summarising the distinct immune ecosystems between two types of recurrent hepatocellular carcinoma
The co-corresponding authors of this article are Prof. Ming Kuang from the Center of Hepato-Pancreato-Biliary Surgery, The First Affiliated Hospital of Sun Yat-sen University, Prof. Dan G. Duda from Harvard Medical School, and Prof. Qiang Zhao from the Organ Transplant Center, The First Affiliated Hospital of Sun Yat-sen University. And the co-first authors are Shuling Chen, an associate chief physician from The First Affiliated Hospital of Sun Yat-sen University,Cheng Huang and Huichuan Sun, professors from Zhongshan Hospital, Fudan University, Shanghai,and Ph.D. candidate Guanrui Liao and Associate Researcher Yubin Xie from The First Affiliated Hospital of Sun Yat-sen University. Based on the HCC specimen library of The First Affiliated Hospital of Sun Yat-sen University, Professor Ming Kuang's team has published a number of high-quality translational precision researches in recent years, starting from important clinical difficulties and closely combining with advance sequencing technologies, with the results published in the journals Annals of Oncology, Gut, Clinical Cancer Research, etc.
Link to the article: http://dx.doi.org/10.1136/gutjnl-2022-328428
Related News
-
China DailyOct 24, 2024
SYSU researchers used AI to identify new RNA viruses
-
China DailyOct 24, 2024
Sun Yat-sen University Institute to boost East-West exchanges
-
Xinhua NewsOct 23, 2024
SYSU researchers unveil new strategy for cardiac arrest
-
Oct 10, 2024
FLS of SYSU Co-hosted 56th Conference of JEINCS
-
Oct 10, 2024
High-level forum on national strategic needs and high-quality development of foreign language disciplines held at SYSU



