Solvent-Dependent Asymmetric Synthesis of Chiral 3,3-Disubstituted Isoindolin-1-ones by CpRhⅢ-Catalyzed C-H Activation
Soutce: School of Chemistry
Written by: School of Chemistry
Edited by: Wang Dongmei
3,3-Disubstituted isoindolin-1-one bearing a tetrasubstituted stereogenic carbon at the 3-position is a common skeleton in natural products and drug candidates, such as inhibitor for microsomal triglyceride transfer protein (MTP) and potential drug for treatment of cardiac arrhythmias. Many elegant methods have been developed for the purpose of enantioselective construction of chiral 3,3-disubstituted isoindolin-1-ones either by organocatalysis or by transition-metal catalysis. However, the substrates were mainly limited to 3-hydroxyisoindolinones, alkenyl benzamides, and N-halobenzoyl indoles, which greatly limits the structural diversity of products. Transition-metal catalyzed C-H activation has shown great potential in the synthesis of 3,3-disubstituted isoindolin-1-ones, being able to overcome some shortcomings of above methods. However, its asymmetric counterpart remains extremely rare and highly challenging.
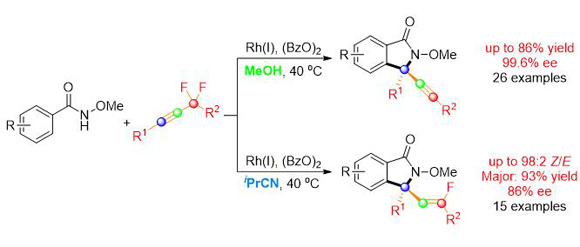
Solvent-dependent enantioselective construction of chiral 3,3-disubstituted isoindolin-1-onesby CpRhⅢ-Catalyzed C-H Activation
Recently, the research group of Professor Jun Wang from the Department of Chemistry at Sun Yat-sen University reported a solvent-dependent enantioselective syntheses of alkynyl and monofluoroalkenyl isoindolinones via asymmetric CpRhⅢ-catalyzed C-H activation. In MeOH the alkynyl isoindolinones were formed, while in iPrCN the monofluoroalkenyl isoindolinones were produced. Mechanistic studies showed that the reaction went through the same E-alkenyl rhodium intermediate in different solvents, furnishing alkynyl isoindolinones by an unusual anti-β-F-elimination in the MeOH system, but monofluoroalkenyl isoindolinones upon protonation in the iPrCN system. Moreover, it was found kinetic resolution process took place in this reaction. Despite achieving moderate enantiocontrol ofchiral allene intermediate, high enantiopurities of Z-monofluoroalkenyl isoindolinonesand alkynyl isoindolinoneswere obtained via a single or two sequential kinetic resolution processes. This paper was published in peer-reviewed journal Angew. Chem. Int. Ed. (Teng Li, Chao Zhou, Xiaoqiang Yan, and Jun Wang*. Solvent-Dependent Asymmetric Synthesis of Alkynyl and Monofluoroalkenyl Isoindolinones by CpRhⅢ-Catalyzed C-H Activation. Angew. Chem. Int. Ed. 2018, 57, 4048.)
This work was supported by National Natural Science Foundation of China.
Paper link: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201712691
