The team of Associate Professor Guosheng Chen and Professor Gangfeng Ouyang made a new breakthrough in the research of protein biohybrid framework
Source: School of Chemistry
Edited by: Tan Rongyu, Wang Dongmei
Proteins can assemble into higherorder superstructures, ranging from dynamic polymers (actin, tubulin, and so on), multi-dimensional architectures (bacterial S-layers, collagen, etc.), and even protein crystal such as cypovirus polyhedral that afford specific biological functions. This biological process inspires scientists to harness proteins (‘‘building blocks’’) and engineer new bio-nanostructures with extraordinary functionality. Metal-coordination, interaction-driven, and programmable DNA-DNA interaction-driven protein assembly, are the more recent advances that show the possibility of designing proteins hybrid materials in a controllable pattern. However, due to the inherent heterogeneity and chemical complexity of a protein, to date, only a few proteins have been assembled into well-defined structure.
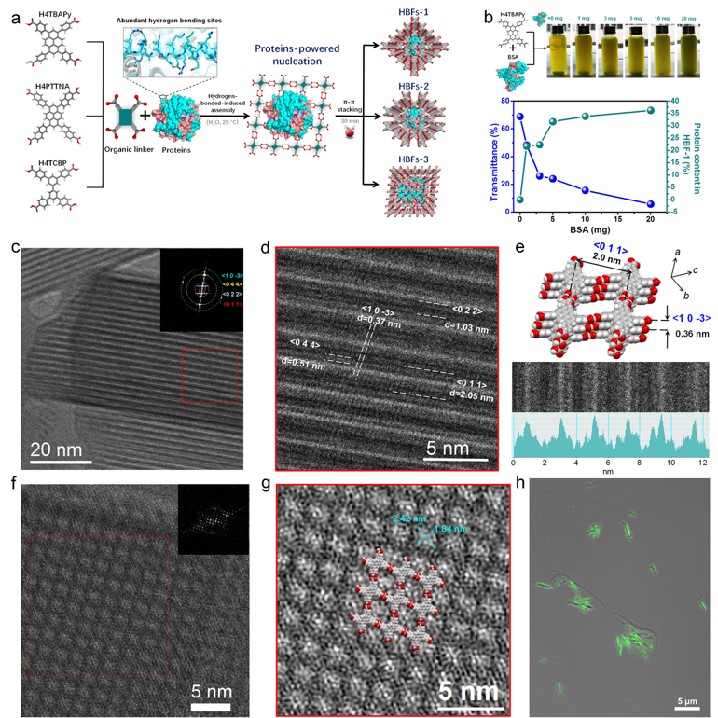
Figure 1. Protein-directed hydrogen-bonded assembly strategy and the cryo-electron microscopy structure of the biohybrid frameworks
In view of these issues, a team led by Associate Professor Guosheng Chen and Professor Gangfeng Ouyang from Sun Yat-sen University reported a novel protein-directed assembly strategy. In this strategy, protein "modules" anchored organic ligands via hydrogen bonding, and then assembled into highly crystalline hydrogen-bonded biohybrid frameworks (HBFs) by strong π-π interaction. Advanced low electron-dose cryoelectron microscopy techniques clearly witnessed the crystallographic structure of hybrid framework at a single-molecular level, and we demonstrated that the proteins were independently and tightly isolated in the crystalline frameworks, with a record-high protein content (as high as 67.4% w/w) in the reported biohybrid framework materials. In addition, the hybrid framework had ultrahigh chemical stability, and its aperture structure and protein confinement tightness were controllable through modulating the organic linkers. When using enzymes as the building block, the obtained enzyme framework displayed notable advantages for biocatalysis compared with the burgeoning enzyme-MOF biohybrids in terms of active ingredient content, robustness, and catalytic efficiency. Our work sheds light on the superiority of the rational assembly of 3D protein hybrid frameworks using hydrogen-bonded interaction, which has enormous potential in biocatalysis, sensing, nanomedicine, etc.
This work has been published in
CHEM entitled “Protein-directed, hydrogen-bonded Biohybrid Framework”. The first author is Associate Professor Guosheng Chen (School of Chemistry) and Assistant Researcher Siming Huang (Sun Yat-sen Memorial Hospital), and the corresponding author is Professor Gangfeng Ouyang (School of Chemistry).
This work is supported by the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province and the Fundamental Research Funds for central Universities. Meanwhile, the research team appreciates the Instrumental Analysis and Research Center of Sun Yat-sen University, Cryo-EM Center of Southern University of Science and Technology, and the Analysis and Testing Center of Southeast University for their support in the relevant testing.
Link to the paper:
https://doi.org/10.1016/j.chempr.2021.07.003