Prof. Xintao Shuai group in the School of Materials Science and Engineering reported important progress in the field of nanodrug antitumor immunotherapy
Source: School of Materials Science and Engineering
Written by: School of Materials Science and Engineering
Edited by: Xu Jia, Wang Dongmei
Recently the team of Prof. Xintao Shuai from the School of Materials Science and Engineering at Sun Yat-sen University published a research paper, entitled "Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-κB inhibitor for antitumor immunotherapy”, in
Science Advances (IF=12.8). Prof. Xintao Shuai is the corresponding author, and Ph.D. candidate Zecong Xiao is the first author.
Nowadays, immune checkpoint blockade is emerging as one of the most appealing and effective means in cancer immunotherapy. In particular, blockade of the PD-1/PD-L1 axis has shown great promise in clinical therapy for patients with melanoma and other types of cancers. Yet, more than half of the patients still cannot benefit from the PD-1/PD-L1 blockade as tumor may develop multiple immune evasion mechanism. Meanwhile, tumor-targeted drug delivery with nanocarriers is based on an early finding that solid tumors may show an enhanced permeation and retention (EPR) effect to macromolecules and nanoscale objects. However, recent studies have found that passive tumor accumulation of nanomedicines is often much less effective than expected because the EPR effect is heterogeneous in humans and may not exist in some tumors. Therefore, research on different drug delivery strategies is of great significance and becoming a hot spot nowadays.
The team of Prof. Xintao Shuai developed a new method to address the two major challenges in cancer immunotherapy. In consideration that the circulating PD-1+ T cells can bind aPD-1 in bloodstream and then traffic actively down chemokine gradients to sites of inflammation or tumors, they proposed an unusual strategy not only applying nanocarrier to deliver aPD-1 for immune checkpoint blockade but also utilizing T cells to deliver an NF-κb inhibitor curcumin (CUR) for antitumor T cell recruitment, which may result in a synergistic tumor immunotherapy (Figure 1). Because of the pH sensitivity, the nanodrug delivered by the tumor-infiltrating PD-1+ T cells may be released in the acidic tumor microenvironment (TME), leaving aPD-1 to block PD-1 on antitumor T cells and meanwhile generating a new CUR-encapsulated nanodrug to act on tumor cells/TAMs for the NF-κb pathway inhibition. Thus, not only the PD-1+ T cells mediate efficient drug delivery to tumor, but also the efficient drug delivery to tumor promotes tumor infiltration of antitumor T cells, thereby resulting in highly effective activation of antitumor immunity.
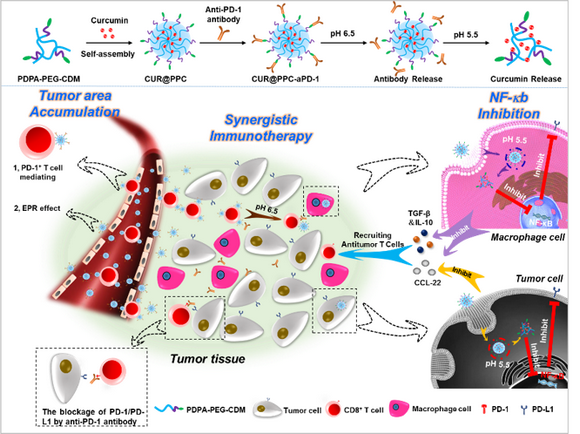
Figure 1. Preparation and tumor-targeted delivery of dual pH-sensitive nanodrug. (Shuai et al., 2020, Sci. Adv.)
This research was supported by the National Basic Research Program of China (2015CB755500), the National Natural Science Foundation of China (51933011), the Natural Science Foundation of Guangdong Province (2014A030312018), and the Guangdong Innovative and Entrepreneurial Research Team Program (2013S086).
Access to this paper:
https://advances.sciencemag.org/content/6/6/eaay7785

