Professor Hui Huang’s team discovered the new potential of longevity protein SIRT6 in vascular calcification
Source: The Eighth Affiliated Hospital
Edited by: Zheng Longfei, Wang Dongmei
Vascular calcification (VC), especially in tunica media, is prevalent in patients with chronic kidney disease (CKD). Previous researches have revealed that VC is a major contributor to major adverse cardiovascular events in CKD and thus is considered an important pathological change in cardiovascular disease. Despite severe clinical consequences, the molecular mechanism underlying VC remains ill defined and no effective therapeutic strategies are currently available to prevent or halt the progression of VC in CKD. Sirtuin 6 (SIRT6) is a member of Sirtuin family, a class III histone deacetylase and a key epigenetic regulator. SIRT6 has a protective role in patients with CKD, however the exact role and molecular mechanism of SIRT6 in VC in CKD patients remains unclear.
Recently, Prof. Hui Huang’s team from the Eighth Affiliated Hospital, Sun Yat-sen University, found a new progress in uncovering the mechanism of VC in CKD, and the new research achievement entitled ‘SIRT6 protects vascular smooth muscle cell from osteogenic transdifferentiation via Runx2 in chronic kidney disease’ was published in
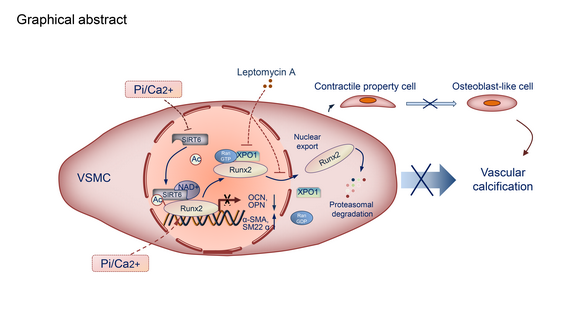
Journal of Clinical Investigation. Using clinical samples from CKD patients, they identified that SIRT6 was decreased in PBMCs and calcified arteries. They demonstrated the effect of SIRT6 inhibition VC in CKD and suppression of osteogenic transdifferentiation in VSMCs both in vivo of WT and SIRT6-Tg mice and in vitro of VSMCs. They identified SIRT6 suppressed the osteogenic transdifferentiation of VSMCs via regulation of runt-related transcription factor 2 (Runx2). Further experiments screening illustrated that SIRT6 bound to Runx2 and deacetylated Runx2, which promoted degradation of Runx2 through the ubiquitin-proteasome system. In addition, they found that ubiquitination and degradation of Runx2 were depend on nuclear export via exportin 1(XPO1). Inhibition of XPO1 partly reversed the protective role of SIRT6 towards VC. In conclusion, SIRT6 prevented VC by suppressing the osteogenic transdifferentiation of VSMCs via promoting Runx2 deacetylation and further nuclear export via exportin 1(XPO1), which in turn caused degradation of Runx2 through the ubiquitin-proteasome system.
Prof. Hui Huang’s group revealed for the first time on the function that SIRT6 prevents VC through post-translational regulation of Runx2 activity and stability. These findings suggest that SIRT6 may be an innovative therapeutic strategy for vascular calcification.
Prof. Hui Huang (The Eighth Affiliated Hospital, Sun Yat-sen University) is the corresponding author. The PhD. student Wenxin Li (The Eighth Affiliated Hospital, Sun Yat-sen University), Dr. Weijing Feng (Nanfang Hospital, Southern Medical University), Dr. Xiaoyan Su (Tungwah Hospital of Sun Yat-sen University) and PhD. student Dongling Luo (The Eighth Affiliated Hospital, Sun Yat-sen University) contributed equally to this work. Professor Baohua Liu at the Shenzhen University Health Science Center provided valuable advice. This research was supported by National Natural Science Foundation of China (No. 8201101103, 81870506, 91849208 and 81670676).
Link to the paper:
https://www.jci.org/articles/view/150051