It is still controversial to what extent neurogenesis occurs in adult primates. On May 30, 2022, the joint teams of Professor Sheng Liu/Yizhi Liu/Mengqing Xiang from Zhongshan Ophthalmic Center of Sun Yat-sen University published the research paper "Single-cell transcriptomics of adult macaque hippocampus reveals neural precursor cell populations" in Nature Neuroscience (impact factor 25). Professors Sheng Liu, Yizhi Liu, and Mengqing Xiang from Sun Yat-sen University Zhongshan Ophthalmic Center and Zhichao Miao from European Bioinformatics Institute are the co-corresponding authors of this paper. Drs. Zhao-Zhe Hao, Jia-Ru Wei, and Dongchang Xiao from Zhongshan Ophthalmology Center of Sun Yat-sen University are the co-first authors of this paper,
This study provides a comprehensive cell atlas of adult primate neurogenesis. Optimized immunofluorescence staining and in vitro culture of neural precursor cells further confirmed the existence of active neurogenesis in the hippocampus. These results provide important medical insights for promoting adult neurogenesis and recovery of damaged nervous systems, such as visual impairment, movement disorders, and memory loss.
Neurons are the basic functional units that make up the nervous system. They can encode, transmit, and decode information, just like the building blocks of a high-rise building. The classical view is that the neural damage is permanent. Neurons cannot regenerate to replace the dead ones. Glaucoma, optic nerve damage, and visual pathway ischemia may lead to the death of optic nerve cells, affecting visual function and even causing blindness. The death of dopamine neurons can lead to Parkinson's disease, causing patients to lose motor function. Alzheimer's disease causes the death of the neurons related to higher cognition and memory, resulting in a decline in memory and cognitive function. Currently, there is no effective treatment for these diseases.
The dentate gyrus of the hippocampus is one of the two brain regions in adult mammals that continuously generate new neurons. Neural stem cells in the mouse hippocampus proliferate and differentiate into the newborn neuron, integrate into the neural circuits and play physiological functions. Whether there is robust neurogenesis in the adult primate hippocampus has been controversial. A systematic study of adult neurogenesis in primates can be of important medical significance for the development of the curation for visual impairments, central nervous system movement disorders, and cognitive dysfunctions.
Previous studies on adult primate brains used single nuclei RNA-seq technology, which only detected the nuclei RNAs. In the current study, the researchers optimized and improved the single-cell isolation technology for the primate brain, and for the first time, obtained the whole-cell single-cell RNA-seq atlas for the hippocampus of the adult crab-eating monkey (Macaca fascicularis). The researchers also depicted the transcriptomic profiles, cellular differentiation trajectories, and gene regulatory network (regulon) during the neurogenesis process. Using the combination of classical molecular marker staining, gene regulatory network expression, data integration, and RNA velocity of the transient state of cellular transcripts, the researchers identified the key cell types during neurogenesis, including radial glia-like cells (RGLs), intermediate progenitor cells (IPCs), and neuroblasts (NBs). Identifying these cell types and their differentiation trajectories recapitulated the molecular processes of primate neurogenesis at the single-cell level (Fig. 1).
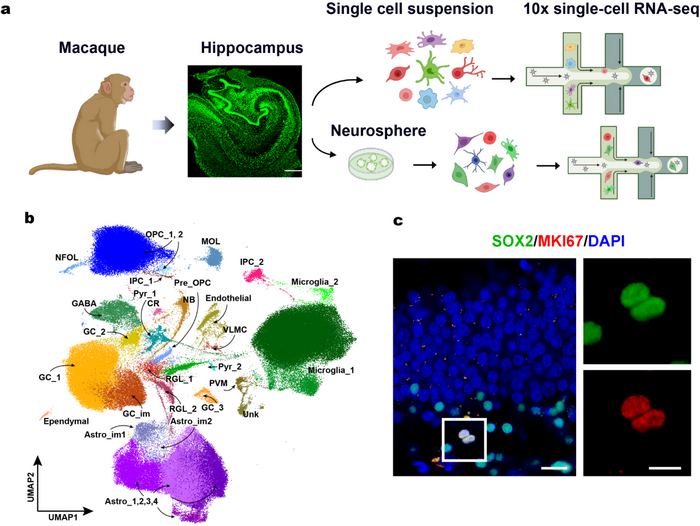
Figure 1. Adult neurogenesis in macaque hippocampus. a. Illustration of the experimental workflow. The fluorescent nissl staining shows the macaque hippocampus. Scale bar, 1mm. b. UMAP visualisation of 207,785 cells from the adult macaque hippocampus forming 34 discriminative cell populations. c. Immunostaining reveals proliferating intermediate progenitor cells.
The researchers found similarities and substantial differences in adult neurogenesis between primates and mice. In particular, through cell differentiation analysis and immunofluorescence staining of the canonical neurogenesis marker DCX, researchers found the presence of abundant immature newborn neurons in the primate hippocampus, in contrast to the very low number of immature newborn neurons in adult mice. This result suggests important differences in neural network plasticity between adult primates and rodents. Studying the shared and different properties during neurogenesis among these species will help to bridge the rich neurogenesis knowledge accumulated in the mouse with the clinical applications in human patients.
The researchers found an important gene, HMGB2 (High-Mobility Group Protein B2), involved in regulating the proliferation of neural stem cells. HMGB2 protein binds to chromatin and participates in the regulation of gene expression. Its expression level is closely related to the transformation process from RGLs to IPCs. The discovery of this gene has important implications for further studies on regulating neural stem cell proliferation (Figure 2).
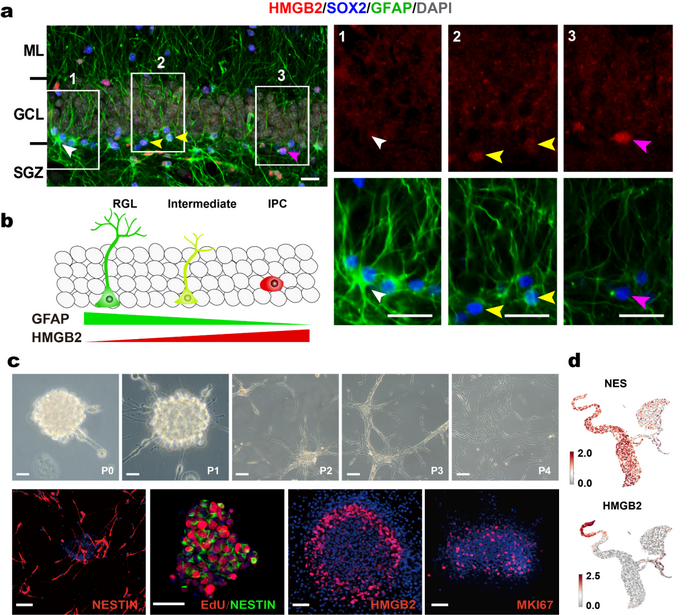
Figure 2. Key regulatory genes and in vitro validation of adult primate hippocampal neurogenesis. a. Immunofluorescence staining proved that HMGB2 gene expression is closely related to the transformation process of RGL to IPC; b. The isolated and cultured adult monkey hippocampal cells have multiple division and passage potential and express typical neural stem cell markers; c. Single-cell sequencing demonstrated that cultured hippocampal cells expressed typical neural stem cell marker genes.
To further validate the existence of neural precursor cells and the neurogenesis in the hippocampus of adult primates, the researchers obtained neural precursor cells and demonstrated their division and passage potency by isolating and culturing adult monkey hippocampal cells. Single-cell sequencing and immunofluorescent staining prove that the hippocampal precursor cells have typical neural stem cell properties and may differentiate into neurons and glial cells. Combined with the data from in vivo experiments, these results further confirm the existence of neural precursor cells in the adult primate hippocampus (Figure 2). These results have a broad application in stem cell therapy and drug screening.
Collectively, this study provides solid evidence and a comprehensive molecular atlas of adult primate neurogenesis.
Link to the paper: https://doi.org/10.1038/s41593-022-01073-x
